The Principles of Quantum Mechanics
Quantum mechanics is afundamental theory in physics that describes the behavior of matter and energy at the atomic and subatomic levels. It's a departure from classical physics, which accurately describes the macroscopic world but fails to explain the phenomena observed at the microscopic scale. The following are some of the key principles of quantum mechanics:
Wave-Particle Duality
One of the most fundamental principles of quantum mechanics is the concept of wave-particle duality. This principle states that particles, such as electrons and photons, can exhibit both wave-like and particle-like properties. For example, electrons can behave like waves when they pass through a double-slit experiment, producing an interference pattern on a detector screen. This wave-like behavior is not observed in classical physics, where particles are considered to be solid objects.
Superposition Principle
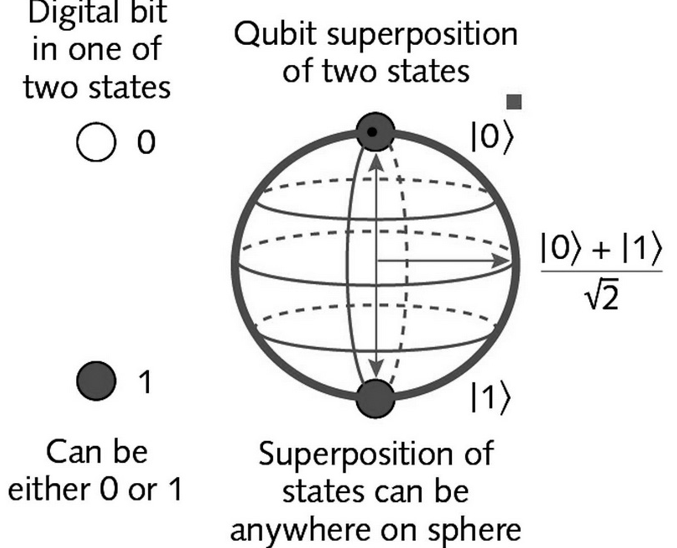
The principle of superposition states that a quantum system can exist in a combination of multiple states simultaneously. For example, an electron can be in a superposition of two different energy states until it is measured, at which point it will collapse into a single definite state. This superposition principle is responsible for many of the unique phenomena observed in quantum mechanics, such as quantum entanglement, where two particles can become correlated in such a way that their properties are linked, regardless of the distance between them.
Uncertainty Principle
Another crucial principle is the uncertainty principle, formulated by Werner Heisenberg. It states that certain pairs of physical properties, such as position and momentum, cannot be known simultaneously with perfect accuracy. The more precisely one property is known, the less precisely the other can be known. This inherent uncertainty arises from the wave-like nature of particles and has profound implications for our understanding of the microscopic world
Quantization of Energy
The concept of quantization plays a crucial role in quantum mechanics. It implies that certain physical properties, such as energy and angular momentum, can only take on discrete values rather than continuous ones. This quantization is observed in the energy levels of atoms and molecules, where electrons can only occupy specific energy states. The concept of quantization has profound implications for our understanding of the stability of matter and the emission and absorption of light by atoms.
Example: When electrons transition between these energy levels, they absorb or emit energy in the form of photons, leading to the emission spectra of elements.Entanglement
- Theory: Particles can become entangled, such that the state of one particle is directly related to the state of another, regardless of the distance separating them.
- Example: If two entangled particles are separated by vast distances, a change in the state of one particle will instantly affect the state of the other, defying classical concepts of locality.
Quantum Tunneling
- Theory: Particles can "tunnel" through energy barriers that they classically shouldn’t be able to pass through.
- Example: This principle is used in modern technologies like tunnel diodes and is a crucial aspect of nuclear fusion in stars.
Probability and Wave Functions
- Theory: The behavior and state of quantum systems are described by wave functions, which provide the probability distribution of a particle’s position, momentum, and other physical properties.
- Example: The square of the wave function gives the probability density of finding a particle in a particular state.
Observer Effect
- Theory: The act of measurement affects the system being measured, collapsing the wave function into a specific state.
- Example: Observing which slit a quantum particle passes through in a double-slit experiment changes the interference pattern, demonstrating how measurement impacts outcomes.
These are just a few of the basic principles of quantum mechanics, and they have far-reaching implications for our understanding of the universe. Quantum mechanics has led to the development of many modern technologies, such as lasers, transistors, and nuclear power. It continues to be an active area of research, with new discoveries and applications being made constantly.
Conclusion
Quantum mechanics challenges our classical understanding of reality and requires us to rethink the nature of particles, waves, and the entire fabric of the universe. It's a field that continues to evolve, bringing new insights and technologies that transform our lives.